Closed System in Thermodynamics
A description of any thermodynamic system employs the four laws of thermodynamics that form an axiomatic basis. For a closed system the second law of thermodynamics is expressed as.

Open System Closed System Isolated System Details System Thermodynamics Mechanical Energy
In any closed system in other words each time a system goes through a thermodynamic process the system can never completely return to precisely the same state it was in before.

. By putting a lid on the saucepan the matter can no longer transfer because the lid prevents the matter from entering the saucepan and leaving the saucepan-This example you will understand when you read open system examples. Second law of thermodynamics. Chapter 4 ENERGY ANALYSIS OF CLOSED SYSTEMS PROPRIETARY AND CONFIDENTIAL.
29 b is the system boundary T is the absolute temperature Q is the rate of energy transfer by heat and S gen is the amount of entropy generated by system. External force and torque on a system are zero. There are four laws which govern the thermodynamic systems phenomena they are.
The second law of thermodynamics for steady-flow system may be expressed as. This is one definition used for the arrow of time since entropy of the universe will always increase over time according to the second law of thermodynamics. The Universe The System The Surroundings.
Closed System In a closed system there is no exchange of matter but exchange of energy is possible between system and the surroundings Fig. Open System In an open system the mass and energy both may be transferred between the system and surroundings. By Ahmed G.
Thermodynamics Chemistry Chapter 6 Important Terms and Definitions System. The term equilibrium in thermodynamics appears in a different context. For example a gas inside a closed rigid container completely insulated from its.
The first law of thermodynamics is a version of the law of conservation of energy adapted for thermodynamic processes distinguishing three kinds of transfer of energy as heat as thermodynamic work and as energy associated with matter transfer and relating them to a function of a bodys state called internal energy. Refers to the portion of universe which is under observation. Work done by a system is positive and the work done on a system is.
The Legendre transform Eulers Theorem on Homogeneous Functions Postulates Equations of state State changes at constant composition Closed control volumes Dynamic systems Open control volumes Gas dynamics Departure Functions Simple vapourliquid equilibrium. The closed-loop system is defined as Feedback from the output to the input is missing in the open-loop control system. Download Free PDF Download PDF Download Free PDF View PDF.
The open system the most general of the three allows mass heat and external work to cross the control boundary. This note describes the following topics. Closed Systems 3 w kJkg work per unit mass w kWkg power per unit mass Sign convention.
Everything else in the universe except system is called surroundings. 62 Open closed and isolated systems. The second law defines the existence of a quantity called entropy that describes the direction.
Closed System Across the boundary of the closed system the transfer of energy takes place but the transfer of mass doesnt take place. A steam turbine is an. First law of thermodynamics.
The balance is expressed in work as all energies into the system are equal to all energies leaving the system plus the change in storage of energies within the system. In a system when there is exchange of energy and matter. The presence of reactants in a closed vessel made of conducting material eg copper or steel is an example of a closed system.
Bahrami ENSC 388 F09 1 st Law of Thermodynamics. The first law specifies that energy can be transferred between physical systems as heat as work and with transfer of matter. To obtain More accurate Control the controlled variable should be fed back and compared with the reference input.
The law of conservation of energy states that. When energy moves into or out of a system the systems internal energy changes in accordance with the law of conservation of mass. The system might be a mechanical device a biological.
2 Flow work of fluid as it exits the system P pressure Ï specific volume P 1 Ï 1 Flow work of fluid as it enters the system dE CV. The state of the entropy of the entire universe as an isolated. Download Free PDF Download PDF Download Free PDF View PDF.
We say the state of a system is an equilibrium state if the macroscopic variables that characterise the system do not change in time. So now Open system 2. Putting a lid on the saucepan makes the saucepan a closed system.
Mass is not fixed. 29 S 2 S 1 1 2 δ Q T b S gen. Lecture notes in Advanced Thermodynamics.
SOLUTION MANUAL SI UNIT PROBLEMS CHAPTER 2 FUNDAMENTALS of. Refrigerator compression of gas in the piston-cylinder assembly are examples of closed systems.

Open Vs Closed Vs Isolated Thermodynamic Systems Thermodynamics Internal Energy Free Energy

Timeline Photos Mechanical Engineers Rocks Facebook Energy System Thermodynamics Mechanical Engineering

Different Types Of Thermodynamic Systems Open System Closed System Isolated S Sponsored Paid Affiliate Thermodynamic Thermodynamics System Chemistry
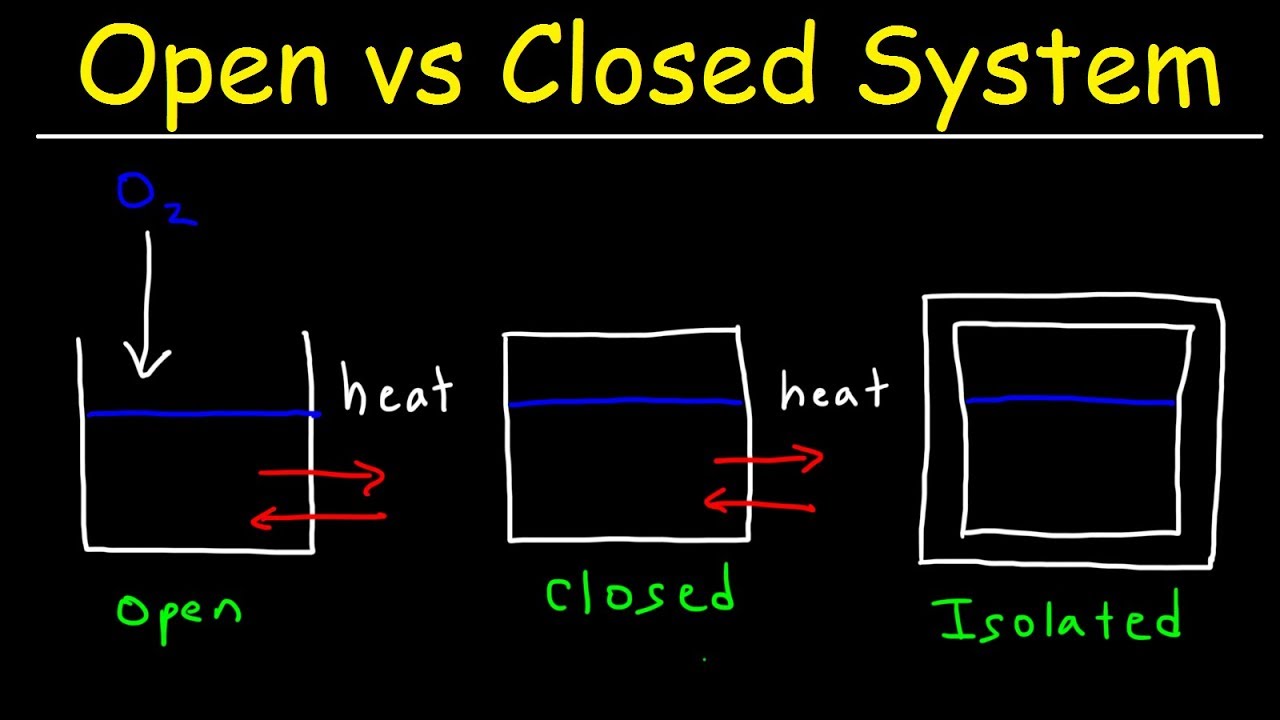
Open System Closed System And Isolated System Thermodynamics Physics
0 Response to "Closed System in Thermodynamics"
Post a Comment